Pigs are important to Iowa. In fact, the corn state puts out more than 2.5 times the amount of pork than its closest rival. Thus, healthy pigs equal a healthy economy in Iowa.
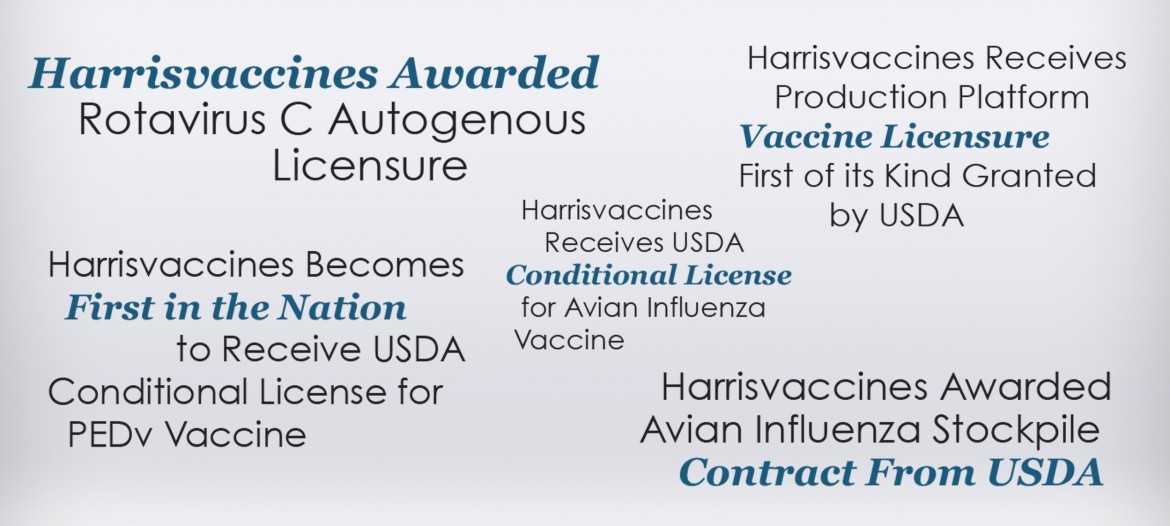
Harrisvaccines is an innovative animal vaccine producer based in Ames, Iowa. The company is a global leader in herd-specific animal vaccine technology, the first vaccine producer to receive a conditional United States Department of Agriculture (USDA) license for a Porcine Epidemic Diarrhea Virus vaccine, and the first company to produce a H1N1 vaccine in face of a pandemic outbreak.
Given their groundbreaking work with herd health and technology, it would be easy to assume Harrisvaccines faced few to no regulatory hurdles. However, because Harrisvaccines utilizes state-of-the-art RNA technology not widely recognized in the industry, they face several regulatory challenges and have a strong need to educate industry stakeholders, policymakers, and regulatory agencies about the safety and efficacy of their products. Therefore, Harrisvaccines employed LS2group’s assistance for public relations, public affairs, strategic planning, and government affairs support beginning in 2012.
The foundation for the LS2group public affairs and government affairs approach was education. Each time antiquated regulations threatened Harrisvaccines’ core business model, LS2group activated call and letter support campaigns and worked closely with policymakers and key influential stakeholders in the agriculture industry to educate them on the important work of Harrisvaccines. LS2group successfully leveraged the team’s wide range of relationships with key stakeholders in the business, agriculture, and political communities to establish positive connections for Harrisvaccines management and familiarize stakeholders with Harrisvaccines’ unique technology.
Through the tactics outlined above, LS2group and Harrisvaccines have been successful in stopping legislation that would jeopardize Harrisvaccines’ core business model. Harrisvaccines has received a number of regulatory approvals from the USDA in recent years that have allowed the company to grow and flourish.